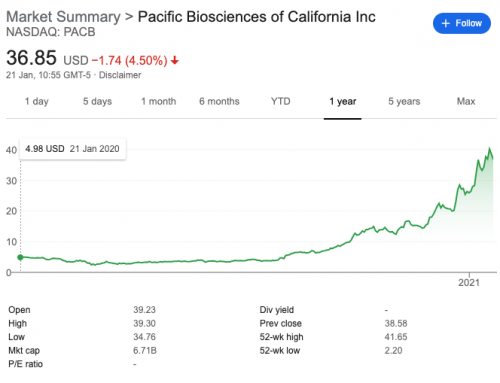
Mammoth Biosciences, one of the two companies developing CRISPR as a diagnostic tool, submitted a medRxiv preprint showcasing their work on a Covid-19 assay.
The SARS-CoV-2 DETECTR assay generate results in 30 minutes from from extracted patient sample RNA. The assay performs simultaneous reverse transcription and isothermal amplification using loop-mediated amplification (RT-LAMP) from RNA extracted from nasopharyngeal or oropharyngeal swabs in universal transport media. On the small number of samples tested so far it has 90% sensitivity and 100% specificity for detection of the coronavirus in respiratory swab samples, corresponding to positive and negative predictive values of 100% and 91.7%, respectively.
Want to know more? Read the coverage on e.g. Biocentury.
This kind of assay may allow testing in the community or even home testing. Good luck to the team at Mammoth in getting this out there!
Figure 1. A CRISPR-Cas12 based assay for detection of SARS-CoV-2

Figure 2. Detection of SARS-CoV-2 in contrived and clinical nasopharyngeal or oropharyngeal swab samples







Leave A Comment