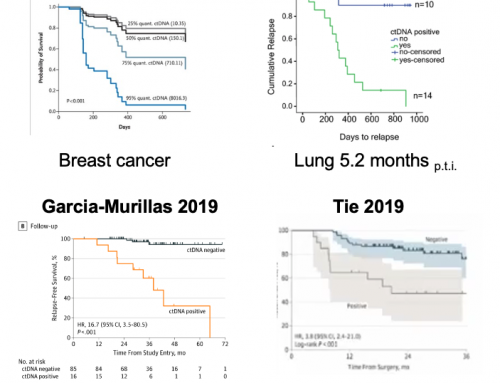
Current liquid biopsy diagnostics, and novel uses such as MRD and Molecular Response analysis, are limited by the amount of ctDNA in a typical patients plasma. ctDNA levels vary across indications (Bettegowda 2014) and generally increase with disease stage. ctDNA is released by tumor cell turnover and is cleared by renal excretion, leading to an incredibly short half-life of around 2 hours. This places limits on whether tests can be applied in early disease, for MRD, Molecular Response or monitoring for recurrence.
In Science this week Victor Adalsteinsson’s group has published their work on novel methods to increase the amount of ctDNA in patients at the time of blood collection. They took a very different approach to others, who have generally focused on improving signal through efficiency of ctDNA capture and/or reducing the background noise of NGS primarily through #coolscience developments in methods such as CAPP-Seq, TAM-Seq, MAESTRO, SaferSeqS, etc.
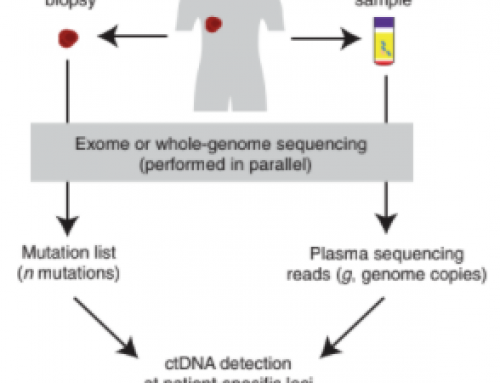
In Priming agents transiently reduce the clearance of cell-free DNA to improve liquid biopsies, newly minted PhD Dr Carmen Martin-Alonso et al present methods to slow the natural clearance mechanisms of cfDNA in vivo and thereby increase signal by increasing the ctDNA concentration in plasma. They accomplish this in pre-clinical model of lung metastases with two priming strategies; nanoparticles to modulate ctDNA clearance by phagocytes and/or a DNA-binding antibodies to prevent nucleosome digestion. See the Fig 1 graphical abstract below.
The paper offers a view into the mechanisms of ctDNA clearance. Their nanoparticle priming (Fig4), with a succinyl phosphoethanolamine–based liposomal agent, inhibited cfDNA uptake by liver-resident Kuppfer cells (part of the mononuclear-phagocyte system) and extended ctDNA half-life. Whilst their antibody binding (Fig 5), with a mouse IgG2a mAb (35I9; Abcam, ab27156), protected double-stranded DNA from DNase1 nuclease digestion.
They demonstrate this has a material impact on ctDNA detection in an MRD setting where detection of 1 or more SNVs was increased up to 60-fold in mice with the lowest tumor burden. However, the overall cfDNA concentration was increased by the administration of liposomes 7-fold, 14-fold, and 28-fold at weeks one, two, and three, respectively in their model. This might suggest that the increase in ctDNA detection was more a function of getting enough molecules to stick around rather than increasing ctDNA concentration relative to cfDNA. As such, if you have very very low ctDNA variants then these may still NOT be detectable excepting with very sensitive assays.
Coming to a clinic…soonish? In the clinic these priming agents would be administered 1-2 hours prior to blood draw so appear to be easy to incorporate initially in a clinical trial of the compunds to demonstrate safety then in ONcology clinical trials and finally in the routine clinic. But before then there’s a lot of work to do e.g. preclinical optimization, formulation, testing, and tolerability studies so we’ll need to wait 3-5 years (at least).
Another company I am aware of in this space is Biocaptiva in Scotland, who are proposing to use plasmapheresis to capture ctDNA from the whole blood volume of cancer patients and capture 100x more cfDNA!






Leave A Comment