Is there such a thing as too many sequencers: Illumina have talked about how they were surprised by the interest in X Ten, and have sold far more units than they initially forecast. The word on the street seems to be that only a few X Ten labs are working at capacity Broad, NYGC, Human Longevity. Illumina have said the reagent pull-though on X Ten has been about $650K/X/year, which is only half of the theoretical $1.2 million/X/year.
Sales of HiSeq 4000 appear not to have been as strong as the 2500 platform was on its launch. NextSeq seems to be popular with almost 1000 units out in the field, especially for NIPT use, but also in medium sized labs wanting their own sequencer. I suspect a fair number of MiniSeq’s are rolling off the production line (although whether they offer good value for money is debatable).
But Illumina’s main reasons for slightly lower than expected performance were clearly lower sales of instruments; and this was particularly so in Europe last quarter. Todd Campbell at The Motley Fool asked an important question about what’s happening in Europe “Europe was [growing] slower than the rest of the world.” but he also poseed the questions “Why? What’s so special about Europe? What are the things that could be going into the reasoning behind Europe growing more slowly than the other parts of the world?” He went on to discuss competition (from Oxford Nanopore) as being a factor, but most telling was something he picked up on from the Illumina conference call when their results were announced at the end of Q2 “Europe is slowing is because of sharing of devices”!
I’d wholly subscribe to the “glut of capacity and increased use of outsourcing” hypothesis. If the glut does not go away, and if labs continue to move to outsourcing then Illumina will sell decreasing numbers of instruments and service contracts, but consumables pull-through should be higher from each box. Ultimately I think this is a win for Illumina as more science will be published using their technology – and that’s really what we all want.
I run a core lab, and I know lots of other people who do in Europe, Africa, and across the world. Sharing Illumina (and other) instruments in core labs has been a part of science for a very long time. It makes good sense scientifically and economically (I know I’m biased). And from where I sit I can see many Illumina sequencers gathering dust (metaphorically speaking), or being run at 25% or lower utilisation. People got the funding to buy these amazing devices; but not the money to staff the lab, to service them, and to fund the projects to run on them. Perhaps worse is the opportunity cost of the lost science; science that could not be done because someone spent money on a sequencer rather than sequences.
Maybe instrument sales have slowed down in Europe because we’ve got wise to this problem, maybe scientists in Europe have seen how great great core labs can be, and that shared devices with high utilisation is a good thing for science in general. But what happens, if I’m right, when the rest of the world realises it has too many sequencers but not as many results as they’d expected, and focuses on buying sequences rather than sequencers?
Will users continue to purchase consumables at an ever increasing rate: Illumina’s business model has been described as “a simple razor and blade model: Illumina makes one-time sales of large machines at lower margins, then provides consumables needed for use in their operation on an ongoing basis.”
A Tweet earlier in the year from Kinghorn Genomics is one of the few public figures I’ve seen for actual sequencing throughput on an X Ten. 1100 genomes in one month is astounding, but still 20-30% short of the 1500 per month figure in Illumina’s specs. Very few owners openly discuss the numbers of samples going through their instruments, and Illumina are very cagey about reagent pull-through in individual labs. It seems pretty clear if X Ten labs simply can’t pull in the required numbers of samples to match Illumina’s specs. But Kinghorn Genomics is at the high end of reagent pull through at < 70% utilisation.
- Total revenue increased by $93.9 million to $1,171.9 million in the first half of 2016; up by 9% over 2015.
- Consumables revenue (63% of total) increased by $128.4 million to $740.1 million in the first half of 2016; up by 21% over 2015 “driven by growth in the sequencing instrument installed base”.
- Instrument revenue (20% of total) decreased by $58.5 million to $243.2 million in the first half of 2016; down by 19% over 2015 “primarily due to lower shipments of our high-throughput platforms”.
- Service and other revenue (15% of total) increased by $23.2 million to $179.2 million in the first half of 2016; up by 15% over 2015 “driven by revenue from genotyping services and extended instrument service contracts associated with a larger sequencing installed base”.
What locks us into Illumina: Capital costs are very high in replacing an Illumina fleet, my own lab has around £2 million invested (2x HiSeq 4000, 1x HiSeq 2500, 1x NextSeq, 2x MiSeq) – we couldn’t simply go out and buy machines from another vendor, even if there were one. The real tie in is the infrastructure we’ve built up around the use of Illumina sequencing. Users are unlikely to switch until there is a really good competitor out there…and Life Tech’s SOLiD and Ion Torrent technologies just were not good enough.
Predicting the future: For the future I’m as confident as everyone else that NGS usage is going up, bigger projects, more samples, more sequencing, more data – that’s a great scenario for Illumina. They might be a bit stuck with the next big leap in instrument yields, as this would need to jump significantly to make labs like mine purchase new boxes, and that could land them back in the same position as they were in 2011. If the economic case for a new machine can’t be made then labs will find it hard to get funding for incremental changes. And if Illumina do make a big leap then many labs may prefer to share the infrastructure costs, and aim bring down experimental costs. Where do Illumina go in the research space next if they can’t bring us cheaper sequencing?
Q: What will Illumina announce at J.P.Morgan? A new sequencer? The $500 genome? Nanopores?
The use of NGS in oncology might take ten years to become profitable given the pace at which healthcare systems can adopt new technologies. I know from my experiences of the NHS that a few labs can be leading lights, but the majority need to be dragged into accepting change of almost any kind. Oncology is tough, but is a huge, and highly profitable, market so the effort from Illumina is likely to be worth it. Illumina certainly think so; SeekingAlpha quoted Francis deSouza (Illumina CEO) as saying “We spent a decade selling instruments to researchers who are experts and understand genomics. Now we’re seeing applications take off, which is a much bigger market for us.” Whether the recent stock fall was partly because the markets see the realisation of this “bigger market” as being too much of a future gamble is unclear to me. Verinata, Grail and Helix are really exciting ventures, but how quickly can they add to Illumina’s revenues and profits?
The rapid adoption of NGS in NIPT might shed some light on the future. Verinata is now contributing high single digit percentages to Illumina’s revenues, and this could reach 10% as soon as 2018. I’d highly reccomend anyone who can get access to BBC Player to watch the “A world without Downs” documentary!
I thought I’d finish up with a look to the future; particularly to the other NGS technology that we might be using alongside Illumina routinely by 2020 – Oxford Nanopore. The technology, soon to be “a genome centre in a box”, and possibly iPhone compatible, is starting to gain traction outside of the hardcore fanboys and fangirls like Nick Loman and Josh Quick. Right now it is certainly an unproven, in the commercial sense; closed-community, the MinION is available commercially, but users are generally talking in the Nanopore forum; and niche tool. But R9 makes Nanopore sequencing easy, and the most recent updates from Clive Brown point to a future where we might use Nanopores alongside SBS. If the ONT tech is truly disruptive then there is a future that may be decidedly less longer orange!
I’d not want to forget to mention Pacific Bioscience now that Sequel appears to be getting some traction (over 100 instruments sold since the launch compared to 100-150 RSIIs). And the 50x drop in DNA required is going to make this a tool people with limited sample availability can now consider using.
But we should not forget that Illumina is a company that can deliver on innovation. Whilst Illumina did not invent SBS – Solexa, a small UK company, did; Illumina turned Solexa’s $2.5 million revenues in 2006, into a $100 million business, in one year! Many readers will remember the release of the HiSeq, MiSeq, NextSeq, X Ten – all significant leaps for genomics; and I’m betting they’ve got some pretty cool tech up their sleeves yet.
Finally: Do you think there are too many sequencers out there? Should we focus on buying sequences rather than sequencers? If the majority of users answer yes to these questions then sequencer sales may well continue to decline in the short term. But reagent pull-through on each box should increase, and Illumina’s focus for research sequencing might shift to “blades rather than razors”, on driving utilisation of their instal base up.


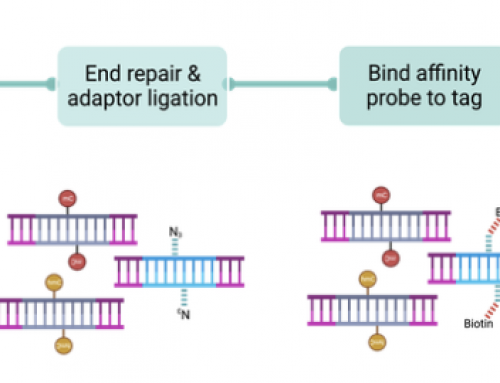






We've recently put together a dashboard showing our throughput at SciLifeLab in Stockholm if you're interested: https://ngisweden.scilifelab.se/file/stockholm_dashboard
This shows output from 5 of our X10 sequencers (the ones based in Stockholm).
Hi Phil,
Nice. I must get you talking to our Bioinformatics team!!!
James.
Great piece thanks James. Certainly some good food thought.
Very interesting article. The key on the consumables will be how proprietary are they, and how soon generic consumables from Thermo Fisher's Life Technologies acquisition can compete on a regular basis.
Hi James. From someone on Wall Street's perspective, it wasn't the $625M to $607M that people cared about. It was the Q4 number going from $685M to $607M. 10% on the year is great however, you're looking at it from historical perspective. Investors care about it from forward perspective. So Q4 growing at 2.5% implies people have to take 2017 number down and that matters more than 2016 overall growth (which includes Q1, Q2, and Q3).
Finally the delta of growth matters more given that we are looking at longer term performance (compounding growth) to gauge value.
For example, We value on 2019 numbers (not on 2016). If growth is accelerating (say from 10% to 13%) 2019 revenue is $3.3B if growth is declining (say from 10% to 7%) 2019 revenue would be $2.9B.
The price of their consumables is too high – 70% profit seems low to me. If they concentrated on what their installed base actually want to do day to day, then they'd never have a shortfall. If users are sharing flowcells to bring costs down then using a core lab – and their expertise – becomes the smarter choice. More people making that choice means fewer instruments being sold. I've always advised people in the market for capital equipment to look at the price of consumables before taking the plunge. I think more prospective sequencer customers are doing just that.