Illumina have released new Nextera Exome and Nextera Custom enrichment kits. These combine the rapid and simple sample prep of Nextera to in-solution genome capture and provide a straight-forward two-and-a-half day protocol. Add one day of sequencing on 2500 and you can expect your results in under one week.
I think many people did not expect exome costs to drop so fast. Ilumina’s reduction of 80% was welcomed by many groups who wanted to run high numbers of exomes but could not when faced with the high costs and complex workflow without pre-capture pooling that other products had. Processing exomes is getting much easier on all fronts. Nimblegen compare their EZ-cap3 to Agilent in the EZ-cap v3 flyer, they did not include Illumina in thecomparison but they did use Illumina sequencing. It looks like Illumina can’t lose whatever exome kit you buy!
A sequenced Nextera exome will cost £250 or $400 with 48 sample kits costing about £4000 and a PE75bp lane about £1000. That’s cheaper than some companies are selling their exome or amplicon oligos for!
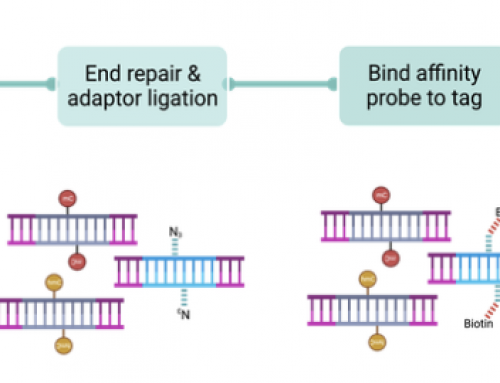
Nextera capture, how does it work: My lab beta-tested the new kits for exome and custom capture. The workflow was as simple as we expected and the data were high-quality. Nextera sample prep still uses just 50ng DNA and takes about three hours. The exome is the same 62MB but now with 12-plex pre-enrichment sample pooling, and still has two overnight captures.
We are still completing processing on our HiSeq of the full data set, but the initial analysis showed similar on and off-target results when compared to TruSeq, there was a higher duplication rate than we would normally see.
Below are three slides I put together for a recent presentation where I discussed our experiences with Nextera capture. They show that Nextera prep is simple (slide1), that QC can be confusing (slide2) but that results are good and costs are low (slide3). As low as £0.01 per exon!
Does Nextera custom capture kill amplicon-sequencing: You can design custom capture kits using Illumina’s DesignStudio (reviewed here). The process is pretty straight-forward and a pool of oligos is soon on its way to you.
Illumina’s Nextera capture only requires 50ng of DNA while TruSeq custom amplicon needs 250ng, so Nextera lets you capture more with less DNA. Small Nextera captures run at very high multiplexing on HiSeq 2500 are likely to be far cheaper than low-plexity amplicon screens on MiSeq. If Nextera custom capture can move to 96-plex pre-capture pooling then the workflow gets even easier (see the bottom of this thread for some numbers supporting this idea).
It will be interesting to see how the community repsinds to Nextera capture. If it takes off then Agilent, Nimblegen, Multiplicom, Fluidigm and others are going to be squeezed. There was a recent paper (MSA-cap seq) from the Institute of Cancer Research describing a low-input 50ng prep for Agilent capture, others like Rubicon and Fluidigm are aiming for the low input space, even single cells.
I am sure we have a lot more to look forward to for the second half of 2012.
Although I still don’t see a $1 sample prep!
PS: One comment I have sent back to the development team is that the protocol requires the same 500ng library input into capture for exome and custom capture. This means the ratio of target to probe is much higher for custom capture and might be reduced. This would allow users to run custom capture with much less PCR. Alternatively if users stick with a 500ng input to capture then they might be able to increase pooling to much higher levels.
Exome capture uses 350,000 probes for 62Mb and 500ng capture input, giving 1.4ng per 1000 probes.
Custom capture uses 6,000 probes per 1Mb and 500ng capture input, giving 83ng per 1000 probes.
Can we run 96-plex pre-capture pooling?
Another thing I would like to see is the impact of completing only one round of capture. I’d assume we’d see a higher number of off-target reads. However saving a day when sequencing is so cheap for a small custom screening panel could be well worth it.










Which planet do you live on where "a PE75bp lane about £1000"?! Oh, the planet where you don't charge staff, overheads or equipment depreciation… 😉
It's called Krypton and we're all superheroes in my lab! Have you seen the prices at BGI!!!
Haha, oh yes 🙂 They make their own reagents in Shanghai sweatshops, and use robots like Star Trek's "Data" to feed the machines – that's why they're cheap. And someone else I know gets a £19M subsidy from BBSRC. Can I come live on Krypton?
Thanks for this update James, helpful. The $1 sample prep issue is now pressing for microbial work, as most of the cost is now sample prep as sequencing itself is trending towards being free.
We also want >=96-plex pre-capture pooling and of course we need DesignStudio to support non-human reference genomes, hopefully this is coming?
Hi Nick, If you want to try the weighted pooling approach (see my next post), perhaps we can get Labcyte to help us set up some experiments? 96 genomes multiplexed wthout any barcodes perhaps?
Hi James, innovative idea, but I don't think it would work for our particular experiments as want to recapitulate the entire sequence for each isolate, whereas I think that approach is more for detecting rare variants??
Hi James. Just to defend amplicon sequencing a little our current amplicon work cost in the region of an order (or 2) in magnitude less. Equally we are just as happy working with pg of DNA as ng… That said it is very exciting the direction these things are going
Dude, you speak like you get paid by Illumina. You are to Illumina as in a fan boy is to apple. Everything Illumina does is right and anything any one else does is either not right or wrong. You fail. I am unsubscribing from your blog post. PS: I m not a lame/naive student. I am a researcher with hand on experience with different NGS systems.
Ouch! I can understand your point of view and would love to have a discussion about the pro's and con's of Illumina vs other technologies. However I don't agree that I am saying Illumina does everything right. I have criticised their non-release of sequences for amplicon analysis and their lack of response to issues around PhiX error rate across flowcells. I have also discussed in some detail the great things other companies are doing around Amplicon sequencing and the somewhat prohibitive cost of TSCA.
I am an Illumina user and in my opinion theirs is the best all-round technology for next-gen sequencing available today. I don't think many people would disagree they are market leaders.
My aim in this blog is to discuss the things I think about in my week. If you don't like what I say then I am happy to hear the criticism and I will take it on board. Sorry to hear you're leaving.
This all sounds good until they published the BED file. As of now, they haven't done that in their MyIllumina portal.
My experience with claimed coverage is: Nimblegen > TruSeq > Agilent. I hope this Nextera one can do better than TruSeq
Hi James,
Interesting information on Exome capture. Can you also provide some idea on how much the custom capture kits cost? Say, for 2Mb of target DNA. Thanks.
k
Hi Karthi, The cost is usually dictated by the size of the region and number of samples. You'd need to speak to your local rep as prices can vary so widely.